Epidermolysis Bullosa treated with transplantation of autologous transgenic epidermal stem cells
History:
A 7-year-old patient with generalized junctional epidermolysis bullosa, non-Herlitz type, presented in July of 2015. Since birth, the patient had suffered from blistering of the entire body with particular manifestations in the extremities, back and flanks. The patient was admitted to the burn unit due to an acute exacerbation and risk of sepsis.
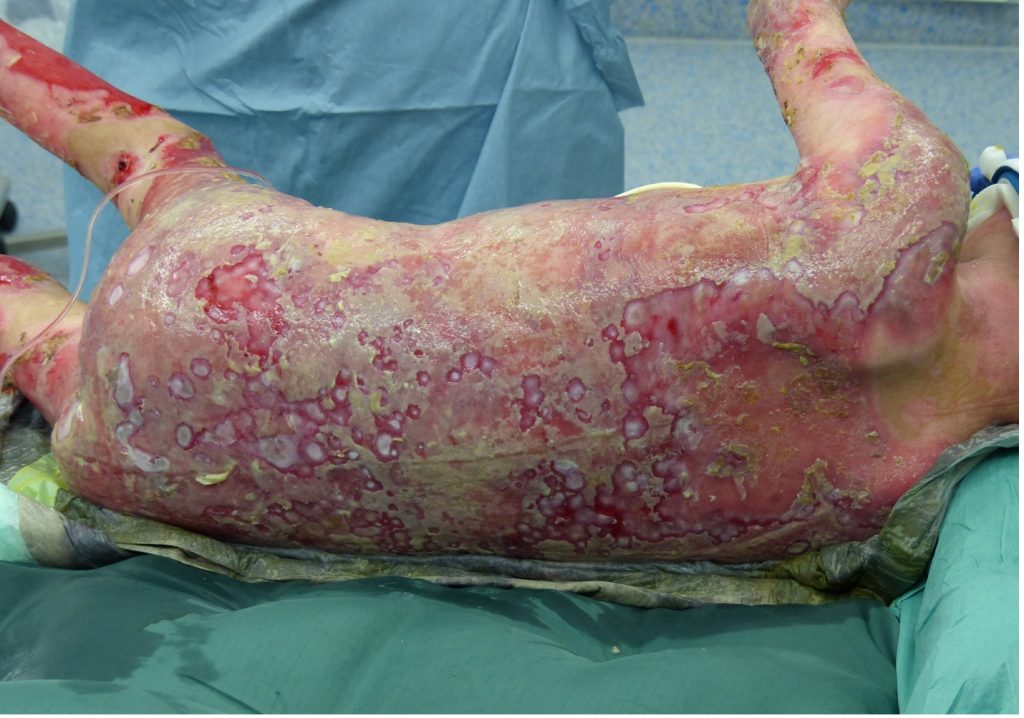
Fig.1. Patient prior to initial debridement following admission
Findings:
Diagnosis:
Differential Diagnoses:
Workup Required:
With an extremely unfavorable short-term prognosis, approval for experimental cell and gene therapy with transgenic epidermal stem cells could be obtained from the relevant authorities as a last resort.
Plan:
Expertise Needed:
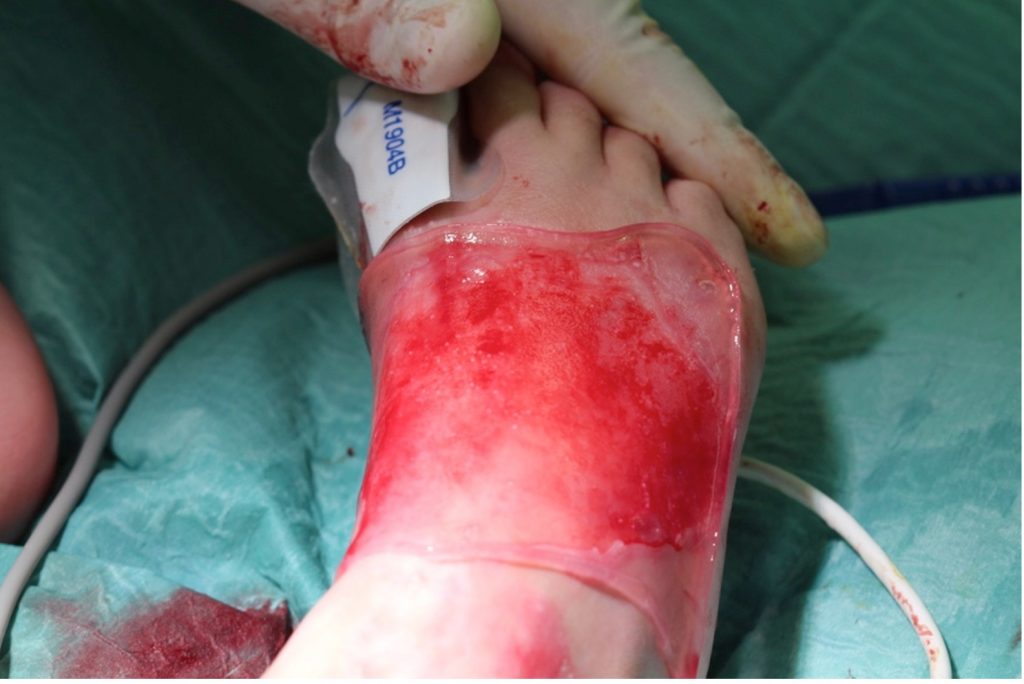
Fig.2. Transplantation of keratinocyte grafts. Cultures are placed on fibrin sheets and placed on the derided wound bed.
Treatment:
In February 2016, the patient could be discharged with a completely regenerated transgenic epidermis in the transplanted areas.
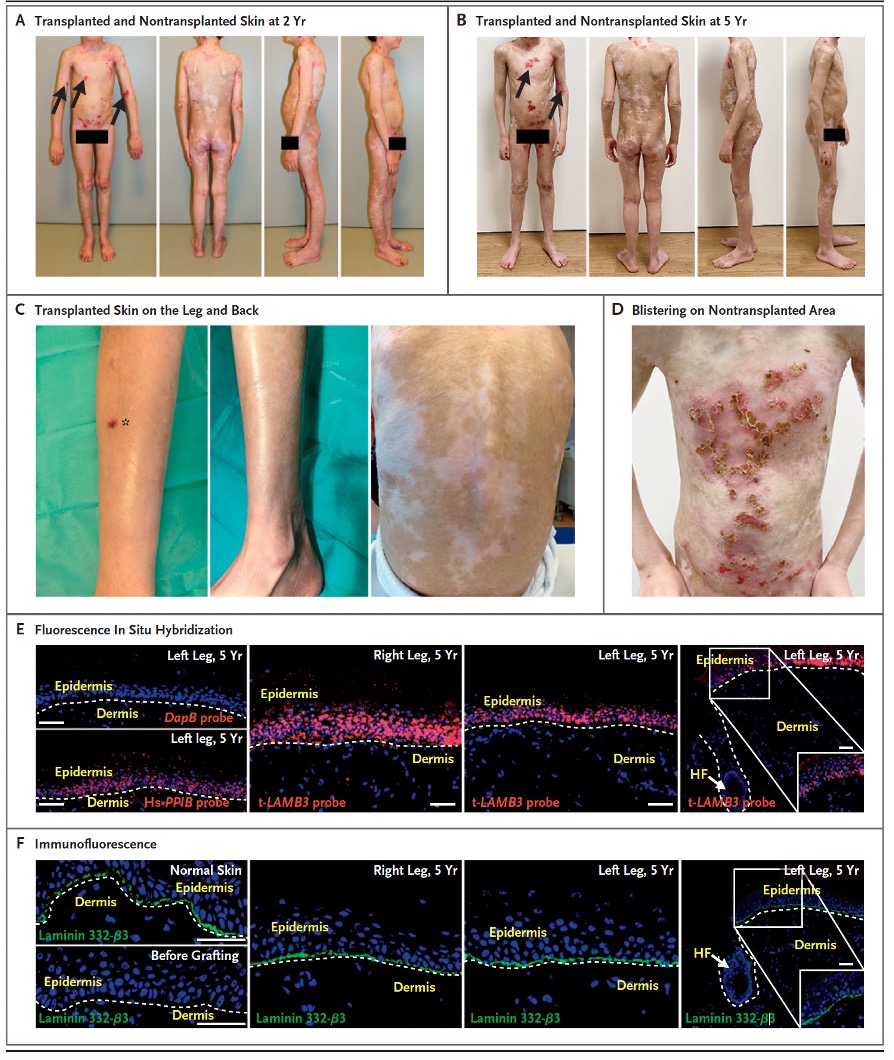
Fig.3. A – D shows macroscopic photographs of healing. E – F shows in situ hybridization and immunofluorescence evidence that the transplanted gene persists. Clinical outcome and evaluation of ex-vivo cultured and genetically modified epidermis after transplantation (Kueckelhaus et al., Transgenic Epidermal Cultures for Junctional Epidermolysis Bullosa – 5-Year Outcomes.
N Engl J Med 2021; 385:2264-2270.). A, B, and C, clinical picture of fully regenerated transgenic epidermis after 2 and 5 years. Blister formation in nontransplanted areas (→). Skin biopsy (*). D, Non-transplanted areas showing blister formation. E, Fluorescence in situ hybridization with vector-specific t-LAMB3 probe. Hair follicle (HF). F, Immunofluorescence analysis with a laminin-332 specific. Dermo-epidermal junction (dashed line).
Follow Up:
Five years after autologous transplantation of ex vivo cultured and genetically modified stem cells, the transgenic epidermis of the patient remained stable without blistering. The transgenic epidermis showed blister-free wound healing, with no appearance of skin contracture. To evaluate the histopathological structure as well as the immunohistochemical components of the transgenic epidermis, a skin biopsy was taken from a transplanted area. Fluorescence in situ hybridization and a vector-specific t-LAMB3 probe demonstrated that the transgenic epidermis was composed entirely of transgenic keratinocytes. Furthermore, immunofluorescence analysis demonstrated that the amount of the dermo-epidermal structural protein laminin-332, was equivalent to that of healthy skin. The transgenic epidermis showed physiological epidermal stratification. Within the dermis, hair follicles and sebaceous and sweat glands could be detected. The absence of laminin-332 from entry to the hair follicle indicated that a stable boundary between transgenic and nontransgenic cells had been established and that stem cells from the hair follicles had not competitively displaced the transgenic epidermal stem cells. Furthermore, the transgenic epidermis was shown to have a physiological amount of correctly localized CD1a-positive Langerhans cells, melanin A antibody-positive melanocytes, and synaptophysin-positive Merkel cells. Collagen IV was present in physiological amounts along blood vessels and the basement membrane. Elastic microfibrils in the stratum papillare region of the dermis appeared reduced, whereas they were detected correctly arranged in the stratum reticulare. The decrease of these elastic microfibrils suggests a slight dermal fibrosis in the area of transgenic skin in JEB-nh patients. The transcription factor p63 could be strongly downregulated after transplantation and showed similar density and localization of healthy skin. The cold-warm sensation improved progressively over the follow-up period reaching normal values after 5 years. Mechanical pain perception in the area of the transgenic skin of the transplanted patient was within the normal range over the entire observation period. A similar trans-epidermal water loss of the transgenic skin and healthy control skin was observed. The levels of melanin were similar to those of a healthy control group. The skin hydration of the transgenic skin was slightly below the threshold values 2 and 3 years after transplantation and reached a similar skin hydration as a healthy control skin after 5 years.. The patient did not require any treatment with moisturizing ointments or drugs following transplantation.

