Lower leg fasciotomy wound treated with with external tissue expander device.
History:
57-year-old patient with history of peripheral arterial disease status post-bilateral iliac artery stenting approximately 1 year ago. The patient has discontinued all his medications and continues smoking.
He presented with an occluded abdominal aorta and iliac arteries. He had profound lower extremity ischemia. He underwent a right axillo-bifemoral bypass using a 8 mm ringed PTFE graft and 4-compartment fasciotomies of bilateral lower extremities.
The patient was taken back to the operating room on postoperative day 3 for bilateral fasciotomy wound washout, partial primary closure and placement of continuous external tissue expanders (Dermaclose, Synovis, Birmingham, AL).
After 5 days of continuous external expansion the wound was primarily closed with 3-0 Nylon vertical mattress sutures at bedside.
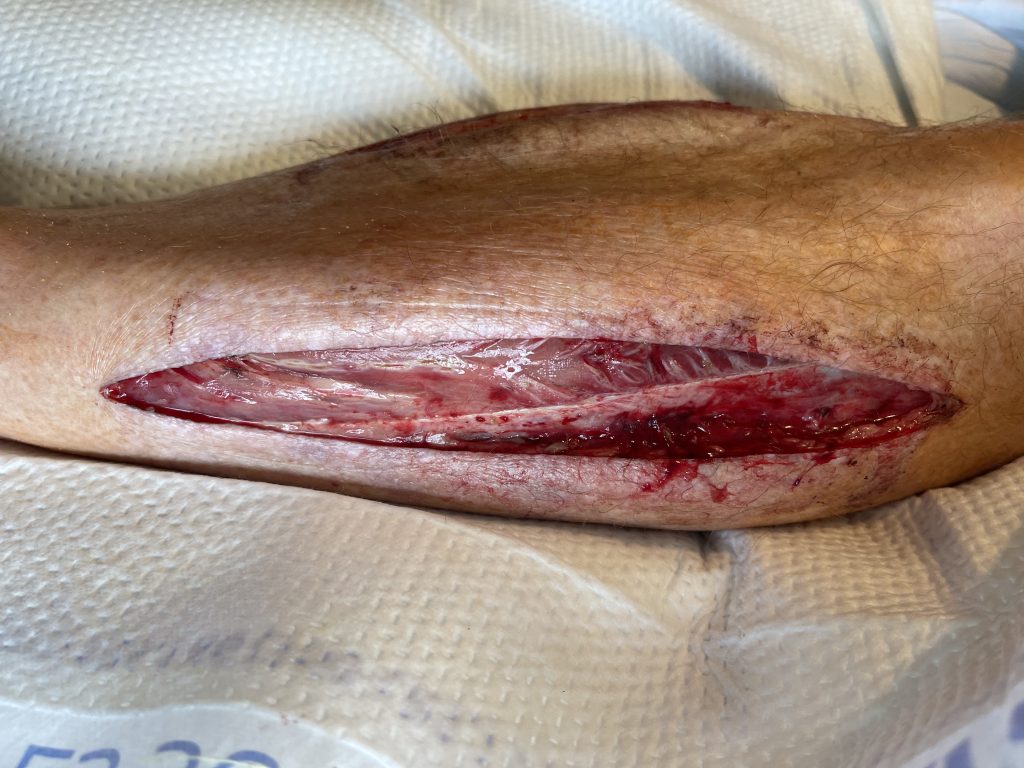
Fig. 1. Left lateral lower extremity fasciotomy wound
Findings:
Diagnosis:
Differential Diagnoses:
Workup Required:
Plan:
The fasciotomy wound should be evaluated and closed as soon as swelling subsides after the index procedure with either primary closure, a skin graft, or delayed primary closure with an external tissue expander.
Expertise Needed:
Make sure to familiarize yourself with the steps of the continuous external tissue expander (Dermaclose).
https://dermaclose.com/wp-content/uploads/2017/02/DermaClose-Quick-Reference-Guide-6-DR-0110_D.pdf
Treatment:
- Plan for fasciotomy wound washout in the operating room as soon as swelling had subsided.
- Plan for primary closure with vertical mattress 3-0 nylon sutures of medial or lateral fasciotomies if possible. If too much tension is encountered use a continuous external tissue expander (Dermaclose) after copious irrigation of the wound. Apply an occlusive dressing over the Dermaclose device.
- At bedside, after adequate pain medication provided, remove the external dressing. Evaluate tissue expansion 48 to 72 hours later by turning tension controller knob clockwise. This can be repeated multiple times at 48 hour intervals until adequate re-approximation of the wound. Typically wounds are re-approximated to within 1 cm in 3 to 7 days and can then be sutured or stapled closed.
- When the fasciotomy wound was tension free with good re-approximation one could attempt primary closure with vertical mattress 2-0 nylon sutures or staples at bedside or in the operating room.
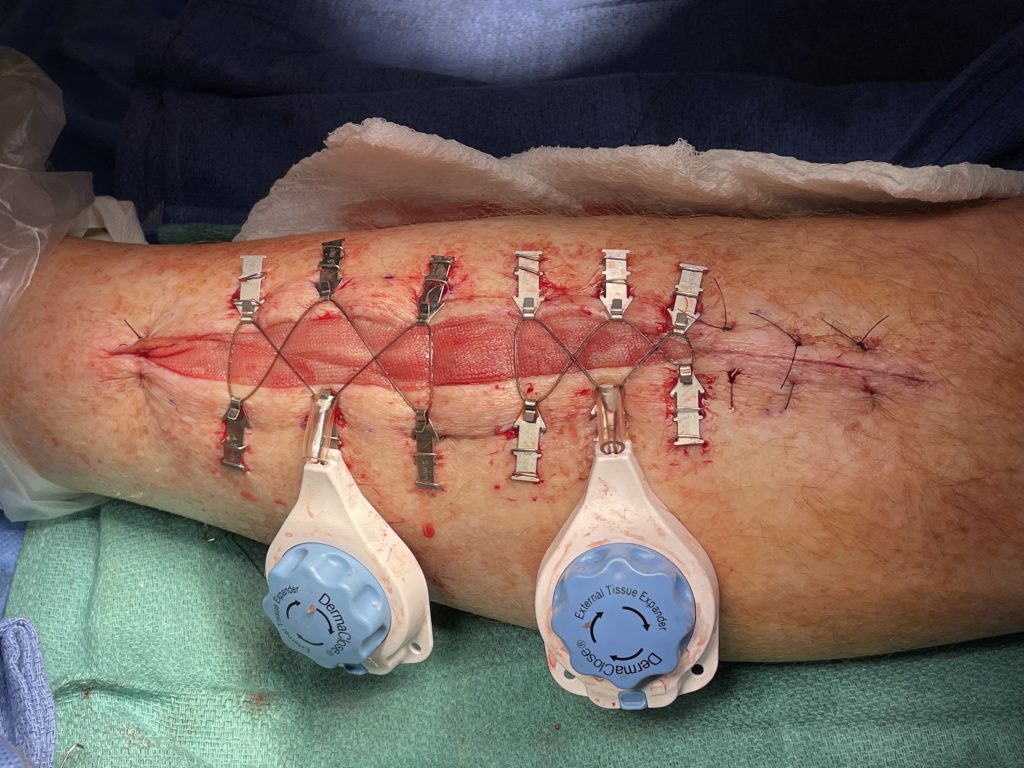
Fig.2. Continuous External Tissue Expander Device (Dermaclose)
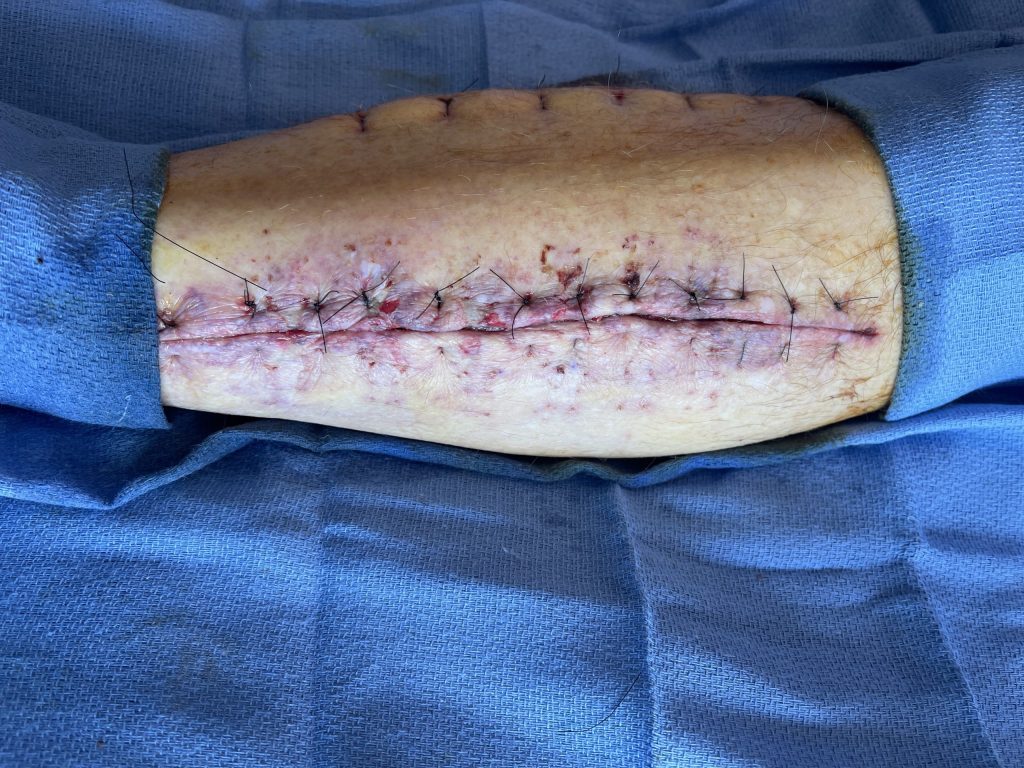
Fig. 3. Lateral left fasciotomy wound re-approximated with 3-0 nylon
interrupted vertical mattress sutures after 5 days of Dermaclose device.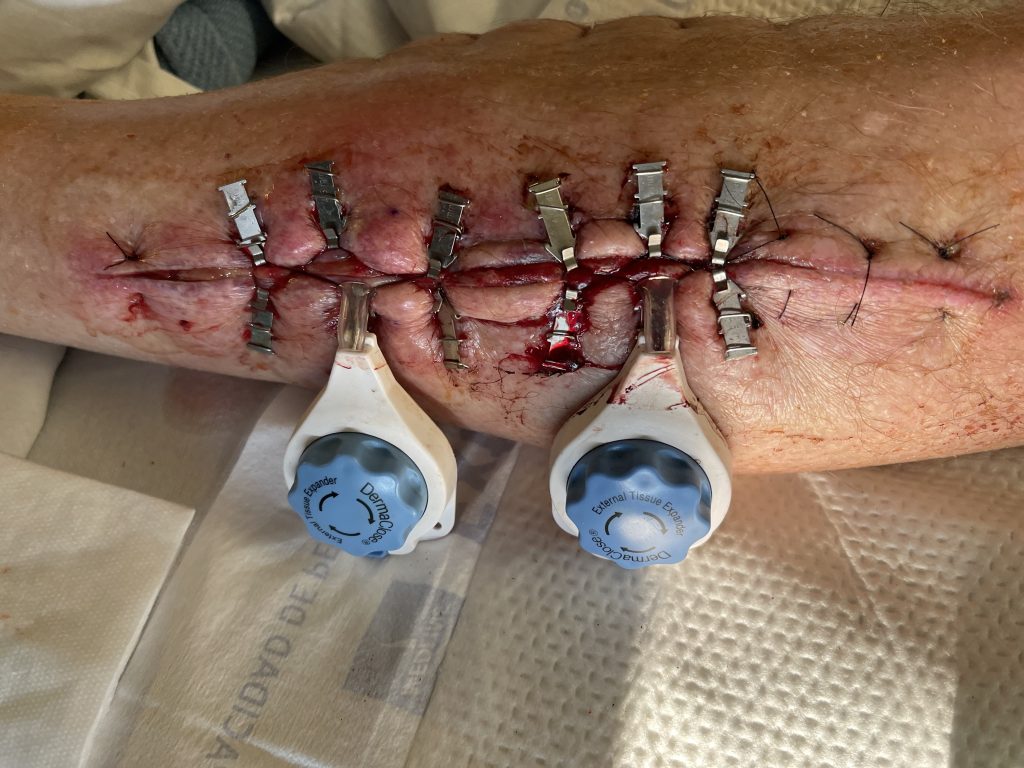
Fig.4. Continuous External Tissue Expander Device (Dermaclose) after 5 days.
Follow Up:
Patients was scheduled for a follow up appointment 2 weeks after primary closure to evaluate the wound and remove the sutures/staples.

